These compounds are commonly used in carbon capture, utilization and storage (CCUS), for purifying and recycling inert gases in solar panel manufacturing, thermochemical energy storage, and producing hydrogen for energy.
These processes are based on reactions where metal oxides gain and lose electrons, known as redox reactions. However, the performance of metal oxides suffers under redox reactions at the high temperatures required for chemical manufacturing.
This is where the Imperial College London technology comes in.
According to Michael High, co-lead author of the study that presents the new solution, metal oxides are key players in a relatively new process called chemical looping combustion (CLC).
CLC is an alternative way of burning fossil fuels that uses metal oxides, such as copper oxides, to transport oxygen from the air to react with the fuel. The reaction produces CO2 and steam, which is condensed to allow the efficient capture of CO2 to prevent it from entering the atmosphere.
By capturing the CO2 that is produced, CLC can help people use fossil fuels in a cleaner way.
However, a key issue that has held back CLC from use on a larger scale is metal oxides’ inability to maintain good oxygen-releasing performance over multiple redox cycles at high temperatures.
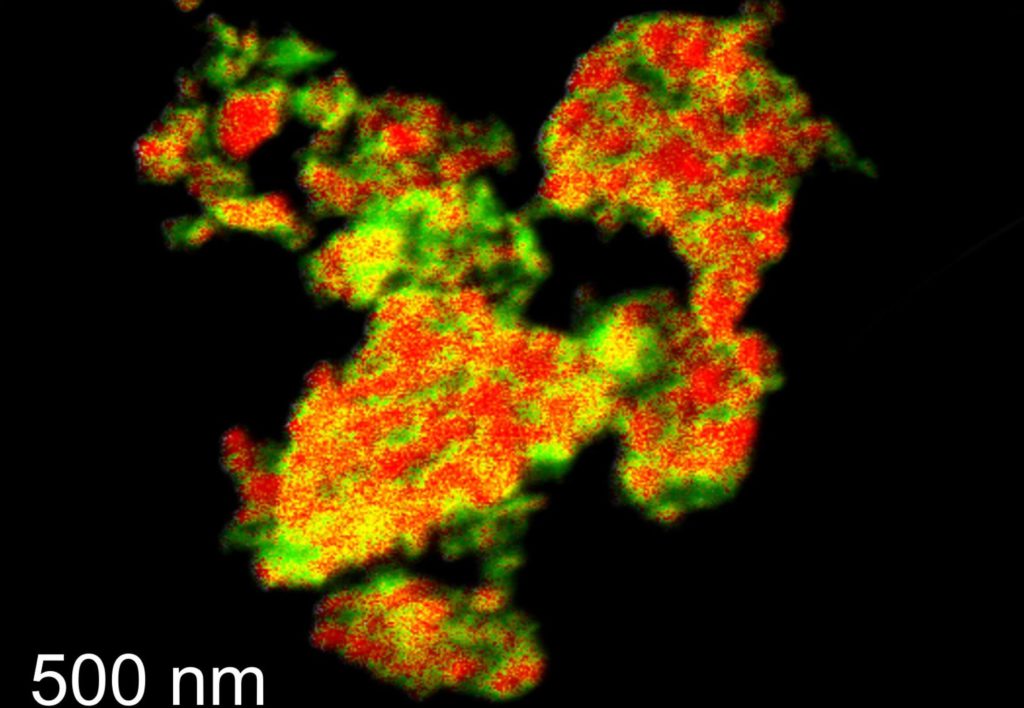
To address this problem, the researchers examined the fundamental structures of the metal oxides used in CLC, reasoning that the precursor chemistry to metal oxides was poorly understood, which limited their rational design.
“To solve the question of how metal oxides maintain their performance, we looked to the basics of the chemical processes involved in CLC,” High said in a media statement. “This is a key example of combining fundamental research and smart design to produce a strategy that’s applicable to a wide range of engineering processes.”

The researcher and his colleagues used an alternative way to engineer the metal oxide structure from a well-known precursor composed of copper-magnesium-aluminum-layered double hydroxides (LDHs).
By tailoring the chemistry of LDH precursors, the scientists found they could produce metal oxides that could still perform well under remarkably high temperatures.
They demonstrated this by putting the oxides through 100 chemical cycles in a widely used type of reactor, known as a fluidized bed reactor, for 65 hours.
Their greater ability to withstand heat means that metal oxides produced in this way can be used to unleash more power from purifying and recycling inert gases like argon in manufacturing solar panels, capturing and storing carbon, chemical energy storage, and producing clean hydrogen. To show this, the group scaled up the production of metal oxides for use in fluidized bed reactors. They found that creating these materials is simple and readily suitable for upscaling using existing industrial manufacturing methods.
“As the world transitions to net zero, we need more innovative industrial processes for decarbonization,” Qilei Song, senior author of the study, said. “To enhance energy security, we must diversify the electricity supply, from renewable energy generation and storage to clean use of fossil fuels with CCUS technologies. Our improved metal oxides hold great potential for use in the energy processes that are helping us reach net zero.”
According to Song, the new solution is already having a global impact on argon recycling in solar panel manufacturing and is expected to help unleash even more power from existing energy technologies that fight the climate crisis.
This post has been syndicated from a third-party source. View the original article here.



