Niobium pentoxide shows promise for speeding up charging of Li-ion batteries
The team points out that during charging, lithium ions move from the positive electrode to the negative electrode, commonly made of graphite. At higher charging speeds, lithium metal tends to accumulate on the graphite’s surface. This effect, known as plating, tends to degrade performance and can cause batteries to short circuit, overheat and catch on fire.
Niobium pentoxide, however, is much less susceptible to plating, potentially making it safer and more durable than graphite. In addition, its atoms can arrange in many different stable configurations that don’t require much energy to reconfigure.

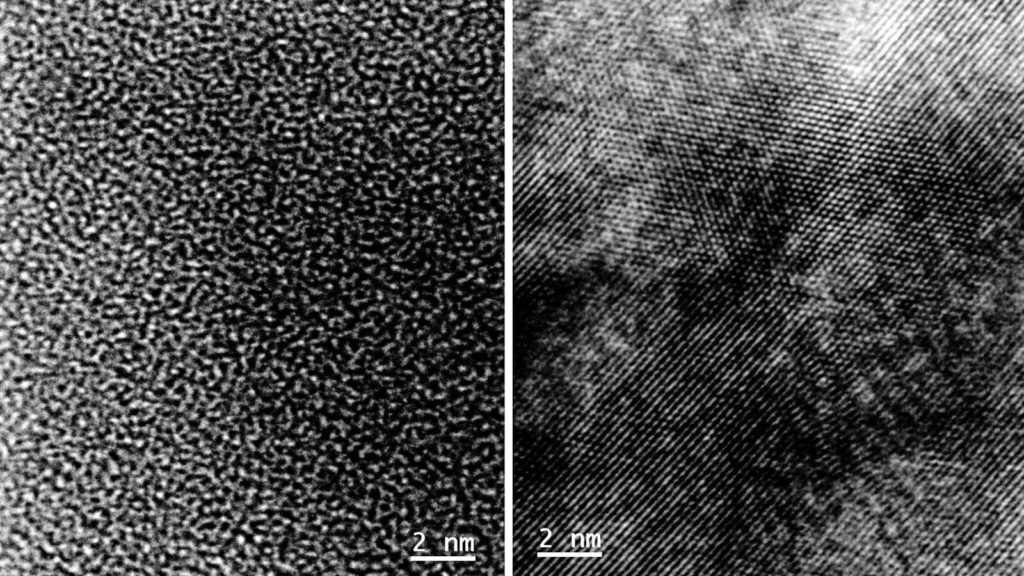
For this study, the researchers built a coin cell – or a small, circular-shaped battery device- with niobium pentoxide as the electrode material. The niobium pentoxide had an amorphous structure—in other words, a disordered arrangement of atoms. When the cell was charged and discharged numerous times, the disordered structure transformed into an ordered, crystalline one. This particular structure had never been previously reported in the scientific literature.
Compared to the disordered arrangement, the crystalline structure enabled easier, faster transport of lithium ions into the anode during charging. This finding points to the material’s promise for fast charging, and other measurements suggest that it can store a large amount of charge.
Because of the complex changes during the charge-discharge cycle, several complementary diagnostic tools were needed for a comprehensive understanding.
From electron microscopy to synchrotron X-ray diffraction
Yuzi Liu, a scientist in Argonne’s Center for Nanoscale Materials and one of the paper’s co-authors, used a technique called transmission electron microscopy to verify the structural transformation from amorphous to crystalline. This technique sends high-energy electron beams through a material sample. It creates digital images based on the interaction of the electrons with the sample. The images show how atoms are arranged.
“Since the electron beam is focused on a small area of the sample, the technique provides detailed information about that particular area,” Liu said in a media statement.
The scientist and his colleagues also used synchrotron X-ray diffraction to confirm the structural change. This technique involves hitting the sample with high-energy X-ray beams, which are scattered by the electrons of the atoms in the material. A detector measures this scattering to characterize the material’s structure.
According to Liu, X-ray diffraction is effective for providing information about overall structural changes across an entire material sample. This can be helpful in studying battery electrode materials because their structures tend to vary from one area to another.
Thus, by hitting the anode material with X-ray beams at different angles, it was possible to confirm that it was uniformly crystalline along the surface and in the interior.
Once this was done, the team used a tool called X-ray photoelectron spectroscopy to evaluate the anode material. They shot X-ray beams into the anode, ejecting electrons from it with a certain energy.
The technique revealed that niobium atoms gain multiple electrons as the cell is charged. This suggests that the anode has a high storage capacity.
“It is very difficult to make the high performance, crystalline niobium pentoxide with traditional synthesis methods, such as those that subject materials to heat and pressure,” the media statement reads. “The unconventional synthesis approach used successfully in this study—charging and discharging a battery cell—could be applied to make other innovative battery materials. It could potentially even support fabrication of novel materials in other fields, such as semiconductors and catalysts.”
This post has been syndicated from a third-party source. View the original article here.